What is CVD SiC Coating?
Chemical vapor deposition (CVD) is a vacuum deposition process used to produce high-purity solid materials. This process is often used in the semiconductor manufacturing field to form thin films on the surface of wafers. In the process of preparing silicon carbide by CVD, the substrate is exposed to one or more volatile precursors, which react chemically on the surface of the substrate to deposit the desired silicon carbide deposits. Among the many methods for preparing silicon carbide materials, the products prepared by chemical vapor deposition have higher uniformity and purity, and this method has strong process controllability. CVD silicon carbide materials have a unique combination of excellent thermal, electrical and chemical properties, making them very suitable for use in the semiconductor industry where high-performance materials are required. CVD silicon carbide components are widely used in etching equipment, MOCVD equipment, Si epitaxial equipment and SiC epitaxial equipment, rapid thermal processing equipment and other fields.
This article focuses on analyzing the quality of thin films grown at different process temperatures during the preparation of CVD SiC coating, so as to select the most appropriate process temperature. The experiment uses graphite as the substrate and trichloromethylsilane (MTS) as the reaction source gas. The SiC coating is deposited by low-pressure CVD process, and the micromorphology of the CVD SiC coating is observed by scanning electron microscopy to analyze its structural density.
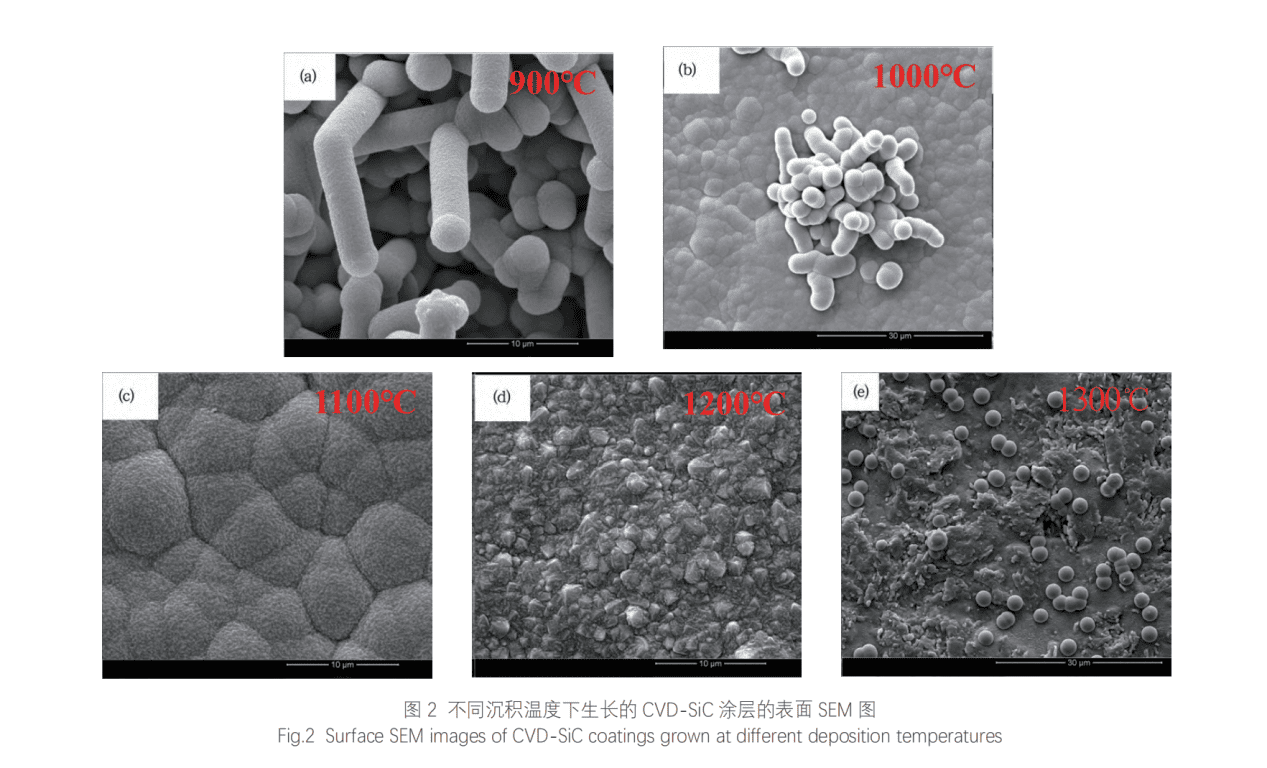
Because the surface temperature of the graphite substrate is very high, the intermediate gas will be desorbed and discharged from the substrate surface, and finally the C and Si remaining on the substrate surface will form solid phase SiC to form SiC coating. According to the above CVD-SiC growth process, it can be seen that temperature will affect the diffusion of gas, the decomposition of MTS, the formation of droplets and the desorption and discharge of intermediate gas, so the deposition temperature will play a key role in the morphology of SiC coating. The microscopic morphology of the coating is the most intuitive manifestation of the density of the coating. Therefore, it is necessary to study the effect of different deposition temperatures on the microscopic morphology of CVD SiC coating. Since MTS can decompose and deposit SiC coating between 900~1600℃, this experiment selects five deposition temperatures of 900℃, 1000℃, 1100℃, 1200℃ and 1300℃ for the preparation of SiC coating to study the effect of temperature on CVD-SiC coating. The specific parameters are shown in Table 3. Figure 2 shows the microscopic morphology of CVD-SiC coating grown at different deposition temperatures.
When the deposition temperature is 900℃, all SiC grows into fiber shapes. It can be seen that the diameter of a single fiber is about 3.5μm, and its aspect ratio is about 3 (<10). Moreover, it is composed of countless nano-SiC particles, so it belongs to a polycrystalline SiC structure, which is different from the traditional SiC nanowires and single-crystal SiC whiskers. This fibrous SiC is a structural defect caused by unreasonable process parameters. It can be seen that the structure of this SiC coating is relatively loose, and there are a large number of pores between the fibrous SiC, and the density is very low. Therefore, this temperature is not suitable for the preparation of dense SiC coatings. Usually, fibrous SiC structural defects are caused by too low deposition temperature. At low temperatures, the small molecules adsorbed on the surface of the substrate have low energy and poor migration ability. Therefore, small molecules tend to migrate and grow to the lowest surface free energy of SiC grains (such as the tip of the grain). Continuous directional growth eventually forms fibrous SiC structural defects.
Preparation of CVD SiC Coating:
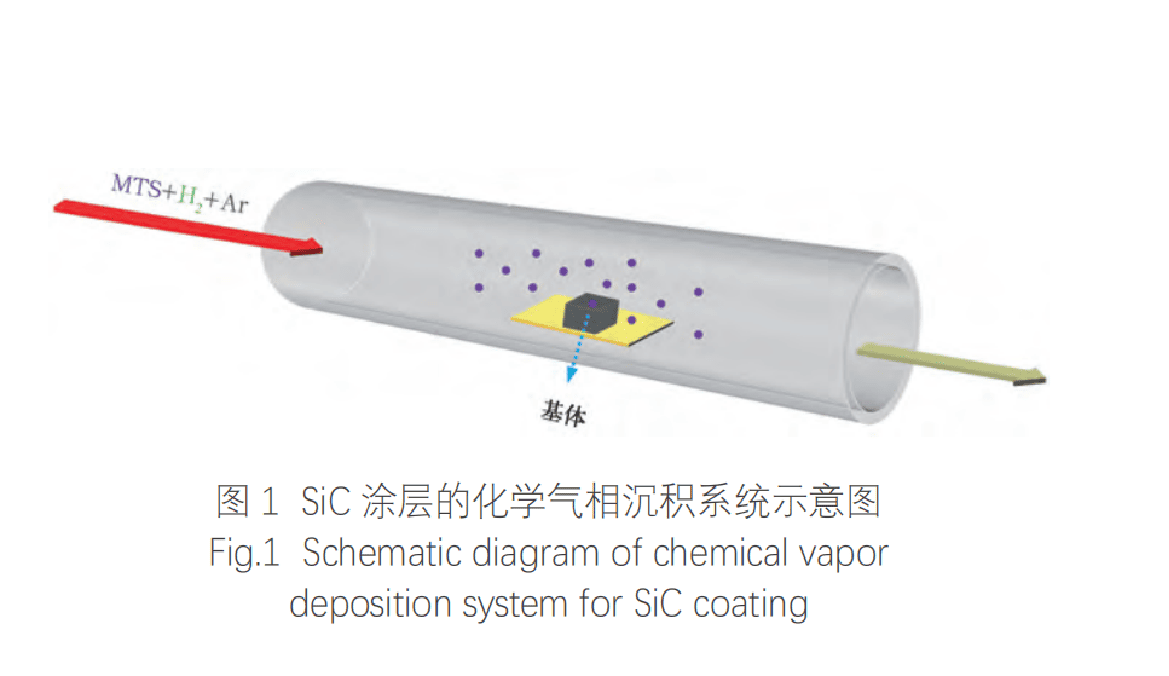
First, the graphite substrate is placed in a high-temperature vacuum furnace and kept at 1500℃ for 1h in an Ar atmosphere for ash removal. Then the graphite block is cut into a block of 15x15x5mm, and the surface of the graphite block is polished with 1200-mesh sandpaper to eliminate the surface pores that affect the deposition of SiC. The treated graphite block is washed with anhydrous ethanol and distilled water, and then placed in an oven at 100℃ for drying. Finally, the graphite substrate is placed in the main temperature zone of the tubular furnace for SiC deposition. The schematic diagram of the chemical vapor deposition system is shown in Figure 1.
The CVD SiC coating was observed by scanning electron microscopy to analyze its particle size and density. In addition, the deposition rate of the SiC coating was calculated according to below formula: VSiC=(m2-m1)/(Sxt)x100% VSiC=Deposition rate; m2–mass of coating sample (mg); m1–mass of the substrate (mg); S-surface area of the substrate (mm2); t-the deposition time (h). CVD-SiC is relatively complicated, and the process can be summarized as follows: at high temperature, MTS will undergo thermal decomposition to form carbon source and silicon source small molecules. The carbon source small molecules mainly include CH3, C2H2 and C2H4, and the silicon source small molecules mainly include SiCI2, SiCI3, etc.; these carbon source and silicon source small molecules will then be transported to the surface of the graphite substrate by the carrier gas and the diluent gas, and then these small molecules will be adsorbed on the surface of the substrate in the form of adsorption, and then chemical reactions will occur between the small molecules to form small droplets that gradually grow, and the droplets will also fuse, and the reaction will be accompanied by the formation of intermediate by-products (HCl gas); When the temperature rises to 1000 ℃, the density of the SiC coating is greatly improved. It can be seen that most of the coating is composed of SiC grains (about 4μm in size), but some fibrous SiC defects are also found, which shows that there is still directional growth of SiC at this temperature, and the coating is still not dense enough. When the temperature rises to 1100 ℃, it can be seen that the SiC coating is very dense, and the fibrous SiC defects have completely disappeared. The coating is composed of droplet-shaped SiC particles with a diameter of about 5~10μm, which are tightly combined. The surface of the particles is very rough. It is composed of countless nano-scale SiC grains. In fact, the CVD-SiC growth process at 1100 ℃ has become mass transfer controlled. The small molecules adsorbed on the surface of the substrate have sufficient energy and time to nucleate and grow into SiC grains. The SiC grains uniformly form large droplets. Under the action of surface energy, most of the droplets appear spherical, and the droplets are tightly combined to form a dense SiC coating. When the temperature rises to 1200℃, the SiC coating is also dense, but the SiC morphology becomes multi-ridged and the surface of the coating appears rougher. When the temperature increases to 1300℃, a large number of regular spherical particles with a diameter of about 3μm are found on the surface of the graphite substrate. This is because at this temperature, SiC has been transformed into gas phase nucleation, and the MTS decomposition rate is very fast. Small molecules have reacted and nucleated to form SiC grains before they are adsorbed on the substrate surface. After the grains form spherical particles, they will fall below, eventually resulting in a loose SiC particle coating with poor density. Obviously, 1300℃ cannot be used as the forming temperature of dense SiC coating. Comprehensive comparison shows that if dense SiC coating is to be prepared, the optimal CVD deposition temperature is 1100℃.
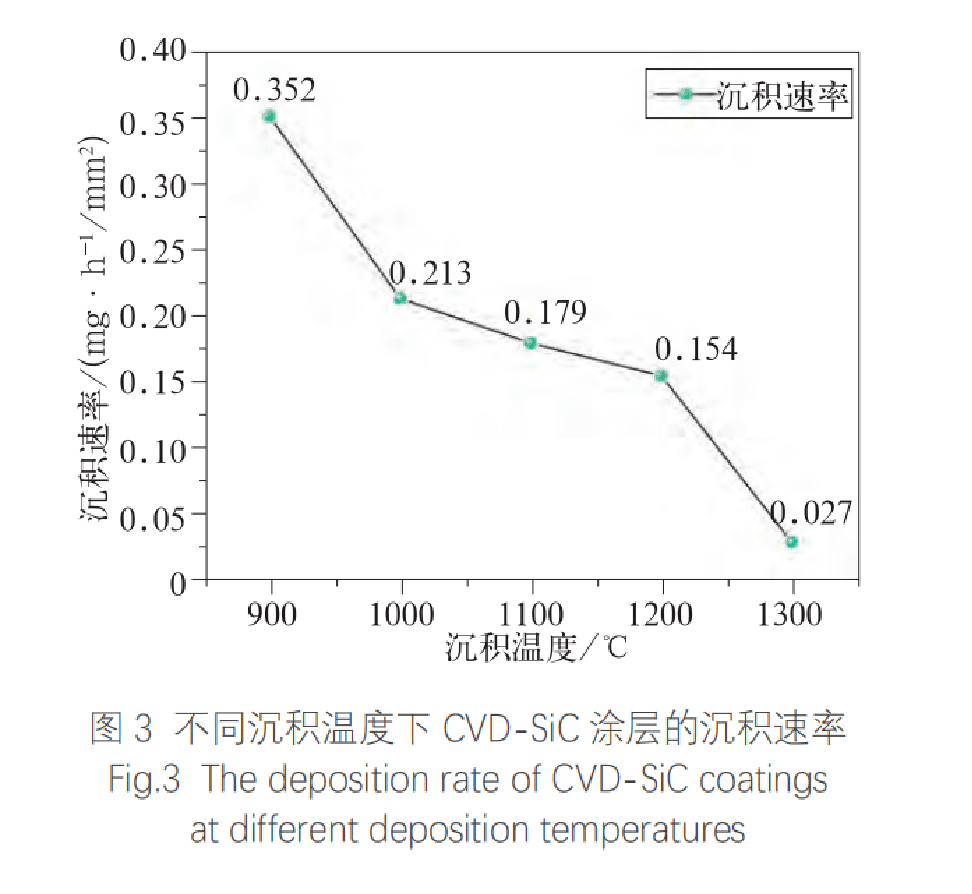
Figure 3 shows the deposition rate of CVD SiC coatings at different deposition temperatures. As the deposition temperature increases, the deposition rate of the SiC coating gradually decreases. The deposition rate at 900°C is 0.352 mg·h-1/mm2, and the directional growth of the fibers leads to the fastest deposition rate. The deposition rate of the coating with the highest density is 0.179 mg·h-1/mm2. Due to the deposition of some SiC particles, the deposition rate at 1300°C is the lowest, only 0.027 mg·h-1/mm2. Conclusion: The best CVD deposition temperature is 1100℃. Low temperature promotes the directional growth of SiC, while high temperature causes SiC to produce vapor deposition and result in sparse coating. With the increase of deposition temperature, the deposition rate of CVD SiC coating gradually decreases.
Post time: May-26-2025